Brick is a running building material in the construction of buildings and structures. Many distinguish only red and white bricks, but its types are much more diverse. They differ both externally (shape, color, size) and properties such as density and heat capacity.
Traditionally, ceramic and silicate bricks are distinguished, which have different manufacturing techniques. It is important to know that the density of a brick, its specific heat capacity and for each type can differ significantly.
Ceramic brick is made from with various additives and is fired. Specific heat ceramic brick is equal to 700 ... 900 J / (kg · deg). Average density ceramic brick has a value of 1400 kg / m 3. The advantages of this type are: smooth surface, frost and water resistance, as well as resistance to high temperatures. The density of ceramic bricks is determined by its porosity and can range from 700 to 2100 kg / m 3. The higher the porosity, the lower the density of the brick.
Silicate brick has the following varieties: solid, hollow and porous, it has several sizes: single, one and a half and double. Average density silicate brick makes 1600 kg / m 3. Pluses of silicate brick in excellent soundproofness. Even if a thin layer of such material is laid, the soundproofing properties will remain at the proper level. The specific heat capacity of silicate brick is in the range from 750 to 850 J / (kg · deg).
The values \u200b\u200bof the density of bricks of various types and its specific (mass) heat capacity at various temperatures are presented in the table:
| Type of brick | Temperature, ° C |
Density, kg / m 3 |
Heat capacity, J / (kg |
|---|---|---|---|
| Trepidny | -20…20 | 700…1300 | 712 |
| Silicate | -20…20 | 1000…2200 | 754…837 |
| Adobe | -20…20 | — | 753 |
| Red | 0…100 | 1600…2070 | 840…879 |
| Yellow | -20…20 | 1817 | 728 |
| Building | 20 | 800…1500 | 800 |
| Facing | 20 | 1800 | 880 |
| Dinasovy | 100 | 1500…1900 | 842 |
| Dinasovy | 1000 | 1500…1900 | 1100 |
| Dinasovy | 1500 | 1500…1900 | 1243 |
| Carborundum | 20 | 1000…1300 | 700 |
| Carborundum | 100 | 1000…1300 | 841 |
| Carborundum | 1000 | 1000…1300 | 779 |
| Magnesite | 100 | 2700 | 930 |
| Magnesite | 1000 | 2700 | 1160 |
| Magnesite | 1500 | 2700 | 1239 |
| Chromite | 100 | 3050 | 712 |
| Chromite | 1000 | 3050 | 921 |
| Fireclay | 100 | 1850 | 833 |
| Fireclay | 1000 | 1850 | 1084 |
| Fireclay | 1500 | 1850 | 1251 |
It is necessary to note one more popular type of brick - facing brick. He is not afraid of moisture or cold. The specific heat capacity of such a brick is 880 J / (kg · deg). Facing brick has shades from bright yellow to fiery red. Such material can be used for finishing and facing work. The density of this type of brick is 1800 kg / m 3.
It is worth noting a separate class of bricks - refractory bricks. This class includes dinas, carborundum, magnesite and chamotte bricks. Refractory bricks are quite heavy - the density of bricks of this class can reach 2700 kg / m 3.
Carborundum brick has the lowest heat capacity at high temperatures - it amounts to 779 J / (kg · deg) at a temperature of 1000 ° C. Masonry from such a brick warms up much faster than from chamotte, but it keeps heat worse.
Refractory brick is used in the construction of furnaces, with a working temperature of up to 1500 ° C. The specific heat of the refractory brick is significantly dependent on temperature. For example, the specific heat of fireclay bricks has a value of 833 J / (kg · deg) at 100 ° C and 1251 J / (kg · deg) at 1500 ° C.
Sources:
- Franchuk A.U. Tables of thermotechnical indicators of building materials, M .: Research Institute of Building Physics, 1969 - 142 p.
- Tables of physical quantities. Directory. Ed. Acad. I.K. Kikoina. M .: Atomizdat, 1976 .-- 1008 p. construction physics, 1969 - 142 p.
- Industrial furnaces. Reference guide for calculations and design. 2nd edition, revised and revised, Kazantsev E.I. M., "Metallurgy", 1975.- 368 p.
The ability of a material to retain heat is evaluated by its specific heat , i.e. the amount of heat (in kJ) needed to raise the temperature of one kilogram of material by one degree. For example, water has a specific heat capacity of 4.19 kJ / (kg * K). This means, for example, that 4.19 kJ is required to raise the temperature of 1 kg of water by 1 ° K.
| Material | Raft- nost, kg / m 3 |
Heat- capacity, kJ / (kg * K) |
Koeffi- heat the wire- w / (m * K) |
TAM mass for heat accumulators 1 GJ of heat at Δ \u003d 20 K, kg |
Relative the village tam mass to the mass of water, kg / kg |
TAM volume for heat accumulators 1 GJ of heat at Δ \u003d 20 K, m 3 |
Relative the village the volume of TAM relative to to the volume of water, m 3 / m 3 |
|---|---|---|---|---|---|---|---|
| Granite, Pebbles | 1600 | 0,84 | 0,45 | 59500 | 5 | 49,6 | 4,2 |
| Water | 1000 | 4,2 | 0,6 | 11900 | 1 | 11,9 | 1 |
| Glauber's salt (sodium sulfate decahydrate) | 14600 1300 |
1,92 3,26 |
1,85 1,714 |
3300 | 0,28 | 2,26 | 0,19 |
| Paraffin | 786 | 2,89 | 0,498 | 3750 | 0,32 | 4,77 | 0,4 |
For water heating systems and liquid heating systems, it is best to use water as a heat storage material, and for air solar systems - pebbles, gravel, etc. It should be borne in mind that a pebble heat accumulator with the same energy consumption compared to a water heat accumulator has a 3 times larger volume and occupies a 1.6 times larger area. For example, a water heat accumulator with a diameter of 1.5 m and a height of 1.4 m has a volume of 4.3 m 3, while a pebble heat accumulator in the form of a cube with a side of 2.4 m has a volume of 13.8 m 3.
The density of heat storage largely depends on the method of storage and the type of heat-storage material. It can be accumulated chemically bound in fuel. In this case, the storage density corresponds to the calorific value, kW * h / kg:
- oil - 11.3;
- coal (standard fuel) - 8.1;
- hydrogen - 33.6;
- wood - 4.2.
During thermochemical heat storage in zeolite (adsorption-desorption processes), 286 W * h / kg of heat can be accumulated at a temperature difference of 55 ° C. The heat storage density in solid materials (rock, pebbles, granite, concrete, brick) at a temperature difference of 60 ° C is 14 ... 17 W * h / kg, and in water - 70 W * h / kg. During phase transitions of a substance (melting - solidification), the accumulation density is much higher, W * h / kg:
- ice (melting) - 93;
- paraffin - 47;
- hydrates of salts of inorganic acids - 40 ... 130.
Unfortunately, the best of the building materials listed in Table 2 is concrete, the specific heat of which is 1.1 kJ / (kg * K), only holds ¼ of the amount of heat that water stores the same weight. However, the density of concrete (kg / m 3) significantly exceeds the density of water. The second column of table 2 shows the density of these materials. Multiplying the specific heat by the density of the material, we obtain the heat capacity per cubic meter. These values \u200b\u200bare shown in the third column of table 2. It should be noted that water, although it has the lowest density of all the materials cited, has a heat capacity of 1 m 3 higher (2328.8 kJ / m 3) than other materials in the table, due to its much larger specific heat capacity. The low specific heat of concrete is largely offset by its large mass, due to which it retains a significant amount of heat (1415.9 kJ / m 3).
- The use of various materials in construction
- Wood
- Brick
The creation of an optimal microclimate and the consumption of thermal energy for heating a private house in the cold season largely depends on the thermal insulation properties of the building materials from which this building was built. One of these characteristics is the specific heat. This value must be taken into account when choosing building materials for the construction of a private house. Therefore, the heat capacity of some building materials will be further considered.
Definition and formula for heat capacity
Each substance, to one degree or another, is capable of absorbing, storing and retaining thermal energy. To describe this process, the concept of heat capacity is introduced, which is the property of a material to absorb thermal energy when heating ambient air.
In order to heat a material of mass m from temperature t beg to temperature t con, it will be necessary to spend a certain amount of thermal energy Q, which will be proportional to the mass and temperature difference ΔT (t con -t beg). Therefore, the heat capacity formula will look like this: Q \u003d c * m * ΔТ, where c is the heat capacity coefficient (specific value). It can be calculated by the formula: c \u003d Q / (m * ΔТ) (kcal / (kg * ° C)).
Conditionally assuming that the mass of the substance is 1 kg, and ΔТ \u003d 1 ° C, we can obtain that c \u003d Q (kcal). This means that the specific heat is equal to the amount of thermal energy that is spent on heating a material weighing 1 kg per 1 ° C.
Back to the table of contents
Use of heat capacity in practice
Building materials with high heat capacity are used for the construction of heat-resistant structures. This is very important for private homes in which people live permanently. The fact is that such designs allow you to store (accumulate) heat, so that a comfortable temperature is maintained in the house for a long time. First, the heater heats the air and walls, after which the walls themselves warm the air. This allows you to save money on heating and make your stay more comfortable. For a house in which people live periodically (for example, on weekends), the high heat capacity of the building material will have the opposite effect: such a building will be difficult to heat quickly.
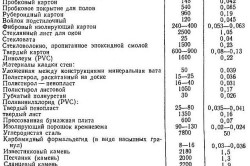
The heat capacities of building materials are given in SNiP II-3-79. Below is a table of the main building materials and their specific heat.
Table 1

Brick has a high heat capacity, so it is ideal for building houses and erecting stoves.
Speaking of heat capacity, it should be noted that heating stoves It is recommended to build from brick, since the value of its heat capacity is quite high. This allows you to use the oven as a kind of heat accumulator. Heat accumulators in heating systems (especially in water heating systems) are used more and more often every year. Such devices are convenient in that it is enough to heat them once with an intense solid fuel boiler, and then they will heat your house for a whole day or more. This will significantly save your budget.
Back to the table of contents
Heat capacity of building materials
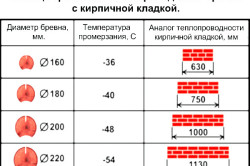
What should be the walls of a private house to match building codes? The answer to this question has several nuances. To deal with them, we will give an example of the heat capacity of the 2 most popular building materials: concrete and wood. it has a value of 0.84 kJ / (kg * ° C), and wood - 2.3 kJ / (kg * ° C).
At first glance, it can be decided that wood is a more heat-consuming material than concrete. This is true, because wood contains almost 3 times more thermal energy than concrete. To heat 1 kg of wood, you need to spend 2.3 kJ of thermal energy, but when it cools, it will also give 2.3 kJ to the space. At the same time, 1 kg of concrete structure is able to accumulate and, accordingly, give only 0.84 kJ.
But do not rush to conclusions. For example, you need to find out what heat capacity will have 1 m 2 of concrete and wood wall 30 cm thick. For this, you first need to calculate the weight of such structures. 1 m 2 of this concrete wall will weigh: 2300 kg / m 3 * 0.3 m 3 \u003d 690 kg. 1 m 2 of a wooden wall will weigh: 500 kg / m 3 * 0.3 m 3 \u003d 150 kg.
- for concrete wall: 0.84 * 690 * 22 \u003d 12751 kJ;
- for wooden structure: 2.3 * 150 * 22 \u003d 7590 kJ.
From the obtained result, we can conclude that 1 m 3 of wood will accumulate heat almost 2 times less than concrete. Intermediate material for heat capacity between concrete and wood is brickwork, in the unit volume of which, under the same conditions, 9199 kJ of thermal energy will be contained. At the same time, aerated concrete, as a building material, will contain only 3326 kJ, which will be significantly less than wood. However, in practice, the thickness of a wooden structure can be 15-20 cm, when aerated concrete can be laid in several rows, significantly increasing the specific heat of the wall.
Sand is considered the most common material., which is used in all spheres of human activity, especially in construction. It is unlikely that there will be a modern building, wherever sand is used as a constituent material. It is used for concrete mix or ordinary mortar for laying a brick wall.
Advantages
Sand has several advantages thanks to which the building has been in operation for many years. The main ones include:
- earthquake resistance;
- it tolerates sudden changes in temperature, from severe frosts to hot climates;
- low compression material, helps to place a heavy base on it, and at the same time additionally absorb the entire structure. This is especially true in areas with frequent earthquakes;
- water permeability, which allows the purification of many liquids;
- wide range of applications in other fields.
For the convenience of determining the heat capacity of the material, in this case sand, ready-made tables are used in which the calculations are given. They are used by builders to carry out calculations.
Thermal conductivity is also important. taken into account when planning thermal insulation works. The selection of the right material is very important, it depends on how much thermal energy you have to spend on heating the finished room.
The main problem is the low heat capacity of the sand material and the finished room, especially if it is a residential building, requires additional thermal insulation. Thermal conductivity depends on the density of the material itself. Another important point is the humidity of the sand.
As indicated in the table below, with its increase, the thermal conductivity of sand material also increases.
Tabular expression of the main parameters of thermal conductivity of sand
This table will help both novice builders and those who are not new to this business, quickly and accurately calculate the required amount of sand material for future development.
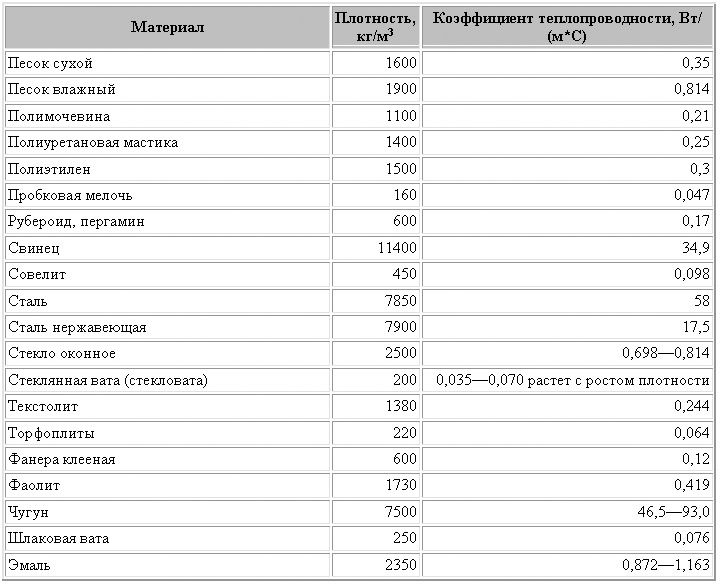
If the construction type of sand is used according to standard GOST sample, then with a mass of 1600 kgm3, the thermal conductivity will be 0.35 W m * deg., and the specific heat is 840 Jkg * deg.
If wet river sand is used, then the parameters will be as follows: a mass of 1900 kgm3 has a thermal conductivity of 0.814 W m * deg, and a heat capacity of 2090 Jkg * deg.
All these data are taken from various manuals on physical quantities and heat engineering tables, where many indicators are given specifically for building materials. So it will be useful to have such a little book at home.
What sand is best used to make concrete?
The widespread use of sand in construction workah allows you to expand the range of applications. is he is a universal tool for the preparation of various types of solution:
- for concrete mixes;
- on ;
- walls;
- laying walls in blocks or bricks;
- pouring bearing pli;
- monolith manufacturing.
You can still list, the main thing is to understand the essence. But in the construction of various kinds of structures, sand with different composition and properties is used.
A unique property, the transition from a loose state to a dense one. Allows you to use this material for protective and natural cushioning of the foundation of the building.
If you select the production component of concrete, then here construction organizations and private builders prefer river sand. Its properties allow you to start using without additional manipulations such as washing, such as career.
The cleanest among the mined sands is the one that is mined from the bottom of existing rivers. It undergoes additional washing treatment and can be used immediately for its intended purpose. The homogeneous mass and the absence of excess impurities make this type of sand the most popular, despite the cost.
A special material and requires an accurate calculation of the proportions of the components, and its quality depends on the presence of clay rocks in the sand. After all, the properties of clay in enveloping sand grains of extracted material, which directly affects the high-quality adhesion of sand with other components of the concrete mix, including cement.
According to the characteristics sand is still divided into classes:
- first grade;
- second class;
- special sands.
Each of these groups is used for concrete products, but only for a narrow circle. So, for example, the first class is used for casting concrete, whose main characteristics are:
- quality;
- high resistance to external influences;
- sudden changes in temperature, including frost resistance.
Sands belonging to the second class are used only for the manufacture of materials that do not require increased moisture resistance, for example, for tiles or facing structures.
Special sand mixtures are necessary for the construction of concrete or reinforced concrete structures. Such mixtures make it possible to strengthen a number of indicators for compression and resistance to atmospheric changes.
For more information about the properties and application of sand, see the video:
Conclusion
Sand is unique natural materialwhich helps to solve many construction issues. The properties of this material allow its use in the construction of complex structures.
And thanks to the low heat capacity, this material is ideal for the construction of rooms where it is required to maintain low temperatures without sudden changes.
From time immemorial sand was used by man, and was considered the most reliable building materialthat nature has created. The variety of types and fields of application helps to think in advance what properties the constructed building will have.
It is generally accepted that any sand is suitable for construction work. But this is not so. First, only special building types. Secondly, it is necessary to take into account their individual characteristics.
The specific gravity and heat capacity of this material play an important role in choosing one of its types, and they will be discussed in this article.
Its specific characteristics depend on the type of material. There are several varieties of it. By origin, it is divided into natural and artificial. The first type, depending on the place of production, has the following varieties:
Career
Quarry sand is mined as a result of the destruction of rocks. Its grains can be from 0.16 to 3.2 mm. Due to the peculiarities of production, it turns out of low quality, since it contains many impurities in the form of clay and dust.
Crushed
It is obtained due to the destruction and grinding of rocks. This process takes place on special equipment, so the extraction of this sand is reflected in its high cost. Because of the resulting irregular shape, the grains of sand are well bonded to each other and other building materials. Adding such material reduces concrete consumption. 
Application: It is used for concrete structures, when filling roads and paths, and also as a filler for dry mixes.
The above varieties of sand vary in color. So, the quarry has a yellow and brown tint, and the river one is cream and gray.
Artificial
It is considered as such, because it undergoes special processing, after which a material is obtained that differs in properties from its original. It is created by crushing natural stones. 
Quartz
It is the most popular of all artificial species. It is obtained by grinding white quartz. After a certain treatment, a homogeneous composition without impurities is produced. This feature of him makes it possible to calculate the exact dimensions of the future design. 
Application: quartz look is widely used in decoration and decorative works, sometimes it is added when creating cement mortarbut this is extremely rare. Usually it is part of paints, fillers and drainage filters.
There is also molding sand, it is used during molding in metal models.
Value determination
This value is equal to the mass placed in a unit volume. Simply put - density. Most often in the reference literature is measured in g / cm 3 or kg / m 3.
The specific gravity of sand depends on the amount of impurities contained in it and the moisture content of the material. A high water content increases the specific gravity per unit volume. Also, this indicator will depend on the storage location of sand, which happens:
- natural occurrence;
- bulk location of material;
- artificial seal.
The same type of sand under these conditions will have different meanings.
According to GOST 8736-77, it is indicated that the specific weight of building sand can range from 1150 to 1700 kg / m 3.
In the table for example, several values \u200b\u200bof its individual varieties are given.
| Type of sand | Specific gravity in kg / 1 m 3 |
| River lining | 1200-1700 |
| 1650 | |
| 1590 | |
| Career | 1500 |
| Nautical | 1620 |
| Quartz | 1600-1700 |
| Wet | 1920 |
Heat capacity
This is the ability of a material to receive, accumulate and retain energy. Heat capacity is an indicator of the thermophysical properties of sand. The ability to heat depends on the chemical composition, structure and amount of material used. Therefore, the overall indicator will depend on its dryness. It is important for cement compositions and for concreting walls.
| Kind of sand | Specific heat in kJ / kg per 1 0 |
| Wet quartz | 2,09 |
| Dry river | 0,8 |
| Career | 0,84 |
| Nautical | 0,88 |
Building sand is a universal material, without which no construction can do. It is an environmentally friendly component of solutions and mixtures. It is steady against burning and is not subject to decay. When choosing its type with high specific thermal conductivity, the concrete structure with it will begin to accumulate heat and an optimal microclimate will be created in the room. This condition can persist for a sufficiently long time. Using sand with a high specific gravity will save on cement.
