Material classification
Traditionally, the materials used in instrumentation are divided into electrical, structural and special purposes. Electrotechnical materials are characterized by certain properties with respect to the effects of electric and magnetic fields; they are developed and produced for use in technology. One of the possible approaches to the problem of classifying the properties of materials is illustrated in Fig. 1.2.
Depending on the ratio of the energy of thermal motion of the particles (atoms, ions or molecules) that form a particular substance, and the energy of their interaction, all materials can be in a gaseous, liquid or solid state. In radio electronics, the fourth state of matter is also used - plasma, which occurs, in particular, after the breakdown of gaseous dielectrics. The transition of a substance from a gaseous state to a liquid state and then to a solid state is accompanied by an increase in ordering in the arrangement of particles in space.
In electronics, materials with both ordered and disordered structures are widely used. In an ordered mono- or polycrystalline solid material, both near and long-range arrangement of atoms (ions) is observed. Disordered condensed materials include those that possess only short-range order in the arrangement of particles in close proximity. Examples of disordered condensed systems include liquids, amorphous and glassy substances, heavily doped semiconductors, disordered semiconductors, and metal alloys.
Fig. 1.2. Material classification
Substances are in gaseous state then, when the energy of thermal motion of particles exceeds the energy of their interaction. Such particles in gases are molecules - less often monatomic (He, Ne, Ar, Kr, Xe, Rn), more often di-, tri- and polyatomic (N 2, O 2, H 2, CO 2, H 2 O, CH 4 , C 2 H 6, etc.). Gas molecules are in chaotic constant motion. Under the influence of external energy influences, some of the molecules are ionized with the formation of ions and electrons.
AT liquid state the energy of thermal motion of the particles forming the substance is comparable to the energy of their interaction. In dielectrics, these particles are molecules that form unstable complexes that continuously decay and re-form. If the molecules are polar, then part of them will be dissociated into positive and negative ions. In liquids short-range order takes place.
Non-ionized gases and non-dissociated liquids are dielectrics. Strongly ionized gases (plasma), melts and aqueous solutions of electrolytes are conductors of the second kind.
AT solid state the interaction energy of atoms (ions) forming a substance significantly exceeds the energy of their thermal motion. Solid materials may be ordered (mono- and polycrystalline), disordered (amorphous and glassy) and mixed.
Single crystals- these are homogeneous, anisotropic bodies, which are characterized by both short and long range order in the arrangement of structural units (atoms, ions) in the entire volume and consist of periodically repeating identical crystalline cells. Single crystals of semiconductor materials are the basis for the manufacture of integrated circuits.
Polycrystalline materials consist of a large number of small crystalline grains (crystallites) grown together, randomly oriented in different directions. Polycrystals are characterized by the presence of both short-range and long-range order in the arrangement of structural units within crystalline grains. Polycrystalline materials include metals, many ceramic materials. Polycrystalline bodies are usually isotropic. However, if ordering is created in the orientation of the crystal (for example, by machining a metal, polarizing ferroceramics), then the material becomes anisotropic. Such bodies with artificially created anisotropy are called textures.
In disordered (amorphous and glassy) bodies, only short-range order takes place in the arrangement of particles (atoms, ions or molecules). They exhibit isotropic physical properties. Glassy materials are solidified liquids that form with decreasing temperature during relatively rapid cooling (increasing viscosity), which impedes the movement of atoms (ions) necessary for the formation and growth of crystals. Glassy materials include glass and resins. An amorphous structure can be observed after disordering (amorphization) of a crystalline material, for example, after its irradiation with accelerated heavy ions. This operation is called ion implantation and is used to introduce impurities into semiconductor single-crystal substrates.
Mixed (glass crystalline) materials are partially crystallized disordered systems. They consist of structural regions with both short-range and long-range order. Partially crystalline structure have many polymers. Glass of certain compositions during aging at elevated temperatures begins to crystallize; due to the small crystals formed, it loses transparency, turning into a glass-crystalline material - sitall.
Chemical bonds between atoms of matter are divided into ionic, atomic (or covalent), metal and molecular. Materials obtained from substances with different bonds vary greatly in their electrical and other properties.
Ionic the bonds are due to the Coulomb attraction of oppositely charged ions. Such bonds are most characteristic of inorganic dielectrics containing ions of opposite signs, for example, Na + - Cl -, Li + - F -.
Atomic (covalent) bonds arise between atoms by the formation of common pairs of valence electrons - one from each atom. Such a pair of electrons is stable as a result of the exchange interaction with the opposite orientation of the spin and corresponding orbital magnetic moments of the electrons. Unlike ionic atomic bonding has a directional character - it is formed in the direction in which the highest density of combined electrons is located. Therefore, substances with atomic bonds are usually hard and brittle. These include crystals of germanium, silicon, diamond, compounds of elements from the middle groups of the table of D. I. Mendeleev - SiC, BN. Atomic bonds are also characteristic of molecules of gases such as H 2, O 2, N 2, as well as molecules of many organic compounds - polyethylene (C 2 H 4) n, polytetrafluoroethylene (C 2 F 4) n, etc. (bonds between individual the molecules of these compounds are molecular).
Metal communication - these are bonds of positively charged metal ions formed by collectivized valence electrons given away by atoms. "Electronic gas" has a cementing effect on the crystalline structure of metals and leads to their high thermal conductivity and electrical conductivity. The undirected nature of the bond determines the high ductility of metals.
Molecularbonds (Van der Waltz) exist in a number of substances between molecules with covalent intramolecular bonds. The intermolecular attraction in this case is due to the coordinated motion of valence electrons in neighboring molecules. At any moment in time, the electrons are as far apart as possible and as close as possible to positive charges. Intermolecular forces are composed of three different types of interaction: orientational (Keesom effect), induction (Debye effect) and dispersive (London effect). In this case, the attractive forces of valence electrons by the positively charged cores of neighboring molecules turn out to be greater than the forces of mutual repulsion of the electrons of the outer orbits. Molecular bonds hold molecules together in solid hydrogen (H 2), nitrogen (N 2), carbon dioxide (CO 2), in many organic compounds - polyethylene, polytetrafluoroethylene, etc. Due to the weakness of molecular bonds, these substances are easily destroyed by the thermal motion of molecules and have low melting and boiling points.
A special type of molecular bond is hydrogen a bond through a hydrogen ion (proton) located between two ions (O - -, F -, Cl -) of neighboring molecules. The hydrogen bond is present in water H 2 O and some organic compounds, as well as in crystals of the type KN 2 PO 4.
By the nature of the interaction with the magnetic field, electrotechnical materials are subdivided into weakly magnetic and strongly magnetic.
Features of the interaction of electro-radio materials with an electric field are the basis of their division into dielectrics, semiconductors and conductors and are discussed in the relevant sections of this study guide.
The properties of materials are divided into functional (service), technological (processing ability) and consumer.
The functional properties of materials can be divided into mechanical, chemical and physical. This division is arbitrary, since mechanics is also a branch of physics. By mechanical properties is meant the behavior of a material under various kinds of loads. The term “physical properties” means the behavior of materials under various types of influences, including heating, electricity, magnetism, light, sound, radiation.
The technological properties of materials include deformability, adhesion, weldability, solderability, etc. Among the consumer properties of materials, the most important are economic, environmental, aesthetic, etc.
We emphasize that the properties of materials can be controlled by changing their composition and structure.
In the particular use of materials, well-defined properties or their combination are crucial. For example, in magnetic devices, the ability of materials to amplify and transform the energy of a magnetic field plays an important role; in devices operating in an electric field, conductivity, polarization and other properties; in cutting tools - hardness, etc. The general requirement for all materials is their cost-effectiveness.
Materials Science. Crib Buslaeva Elena Mikhailovna
1. The subject of materials science; modern classification of materials, the main stages of the development of materials science
Materials science studies the composition, structure, properties and behavior of materials depending on environmental influences. The impact can be thermal, electric, magnetic, etc. Any component of a structure or structure is subjected to loads from other components as well as from the environment.
Classification of materials: metallic, non-metallic and composite materials. Metal materials are divided into non-ferrous metals, powder materials. Non-metallic materials: rubber, glass, ceramics, plastics, ceramic. Composite materials are composite materials, which include two or more materials (fiberglass).
There is a classification of materials depending on the type of semi-finished products: sheets, powders, granules, fibers, profiles, etc.
The technique of creating materials is the basis for classification by structure.
Metallic materials are divided into groups in accordance with the component that underlies them. Ferrous metallurgy materials: steel, cast irons, ferroalloys, alloys in which the main component is iron. Non-ferrous metallurgy materials: aluminum, copper, zinc, lead, nickel, tin.
The basis of modern technology are metals and metal alloys. Today, metals are the most versatile class of materials for use. In order to improve the quality and reliability of products, new materials are required. To solve these problems, composite, polymer, powder materials are used.
Metals are substances that have ductility, gloss, electrical conductivity and thermal conductivity. In technology, all metallic materials are called metals and are divided into two groups.
Simple metals are metals that have a small amount of impurities from other metals.
Complex metals are metals that represent combinations of a simple metal as a base with other elements.
Three quarters of all the elements in the periodic system are metals.
Material science or the science of materials has been developed since ancient times. The first stage in the development of materials science begins with specialized manufacture of ceramics. A special contribution to the development of materials science in Russia was made by M.V. Lomonosov (1711–1765) and D.I. Mendeleev (1834–1907). Lomonosov developed a course in physical chemistry and chemical atomism, and confirmed the theory of the atomic-molecular structure of matter. Mendeleev is credited with developing a periodic system of elements. Both scientists paid considerable attention to the problem of glass production.
In the XIX century. contribution to the development of materials science made F.Yu. Levinson-Lessing, E.S. Fedorov, V.A. Obruchev, A.I. Fersman, N.N. Beleubsky. New materials begin to be produced: Portland cement, new gypsum, cement concrete, polymeric materials, etc.
In mechanical engineering, metals and metal alloys are widely used, which is why metal science is an important part of material science.
Metallurgy as a science arose in Russia in the 19th century, it is the scientific basis for the development of new optimal technological processes: heat treatment, casting, rolling and stamping welding. The combination of high strength and hardness with good ductility, toughness and workability, not found in other materials, was the reason for the use of metals as the main structural material in all areas of technology.
The first established the existence of a connection between the structure of steel and its properties, the outstanding Russian scientist P.P. Anosov (1799–1851), who revealed the long-lost secret of the manufacture and production by the ancient masters of the East of damask steel, which is used for the production of blades. Damask steel Anosov was famous all over the world and even exported abroad. The blades that were made of this steel were characterized by high hardness and toughness. P.P. Anosov is considered the “pioneer” in the production of high-quality steel. He first used a microscope to determine the structure of steel and laid the foundation for studying the regular relationship between the structure and properties of alloys.
The founder of scientific metallurgy D.K. Chernov (1839–1921), who discovered in 1868 the phase transformations in steel. Discovery D.K. Draft critical points a and b (according to the modern designation A1 and A3) revolutionized the knowledge of the nature of metal alloys and made it possible to explain a number of "mysterious" phenomena that occur during the heat treatment of steels.
A huge contribution to the development of the science of metals was made by N.S. Kurnakov, A.A. Baykov, N.T. Gudtsov, A.A. Bochnar, G.V. Kurdyumov, S.S. Shteiberg, A.P. Gulyaev, as well as other Soviet scientists.
Of great importance in the development of metal science and heat treatment were the works of Osmond (France), Seitz, Bane and Mail (USA), Tamman and Hahnemann (Germany).
In the XX century, major advances were made in the theory and practice of materials science, high-strength materials for tools were created, composite materials were developed, the properties of semiconductors were discovered and used, methods for strengthening parts by heat and chemical-thermal treatment were improved.
the author Team of Authors From the book Technical regulations on fire safety requirements. Federal Law No. 123-FZ of July 22, 2008 the author Team of Authors From the book Technical regulations on fire safety requirements. Federal Law No. 123-FZ of July 22, 2008 the author Team of Authors From the book TRIZ Textbook author Hasanov AI1. The subject of TRIZ Hasanov A. I.
From the book Quality Management the author Shevchuk Denis Aleksandrovich1.1. Course subject and objectives One of the main problems facing Russian enterprises today is their successful adaptation to the conditions of a market economy. A solution to this problem is a necessary condition for their survival and further development.
From the book Materials Science: lecture notes the author Alekseev Victor Sergeevich1. Classification of heat-insulating materials During the construction of industrial facilities, civil structures, the accompanying heat supply communications protect from the effects of negative temperatures with the help of various types of heat-insulating materials.
From the book Occupational Safety at Work and in the Learning Process the author Petrov Sergey Viktorovich1.1. The subject of labor protection. The basic concepts of labor protection The subject of labor protectionThe subject of the scientific discipline "Labor Protection" is a system for preserving human life and health in the course of labor activity. Experience shows that any type of human activity should
From the book Metrology, Standardization, and Certification: Lecture Notes author Demidov N.V.1. The subject and tasks of metrology Over the course of world history, a person has had to measure various things, weigh products, count time. For this purpose, it was necessary to create a whole system of various measurements necessary to calculate the volume, weight, length, time
the author Kumanin Vladimir Igorevich3.2. Classification of jewelry materials 3 .Zb the classifier of materials from which jewelry is made is given. Most of them are made of copper-based alloys and precious metals. To a lesser extent, alloys are used on
From the book Materials for Jewelry the author Kumanin Vladimir Igorevich4.1. The main mechanical properties of materials. Jewelry manufacturing is a multi-stage process and always starts with casting, that is, obtaining an alloy in a liquid state, pouring it into a mold, and crystallization. In some cases, the alloy is used in the form
From the book General device of ships author Chaynikov K. N.Chapter VIII. Ship systems § 39. The main elements and classification of systems Ship systems are called a complex of pipelines with fittings, mechanisms, tanks, apparatuses, devices and means of control and control over them. Ship systems
From the book Technology of the Publishing Process the author Ryabinina Nina Zakharovna1.1. Milestones In the encyclopedic dictionary “Book” (Big Russian Encyclopedia, 1998), the profession of an editor is defined as follows: “Editor is a literary worker, a specialist who is professionally engaged in editing.” Actually editing is what
From the book Instrument Engineering author Babaev MA56. Prerequisites for the successful development of modern domestic instrumentation. The main trends in the development of instrumentation Only 20 years ago, one could only dream about the current level of computerization of the country, today all this is a reality. In connection with all these
the author Team of Authors5.1.3. MAIN STAGES OF ELECTRICITY DEVELOPMENT IN OUR COUNTRY In the development of the electric power industry, the following main stages can be distinguished: connecting power plants for parallel operation and forming the first power systems; forming territorial associations
From the book History of Electrical Engineering the author Team of Authors5.3.1. BASIC STAGES OF DEVELOPMENT OF ELECTRIC NETWORKS The chronology of the development of power lines (power lines) of three-phase alternating current in Europe and the USA is well known. In fig. 5.7 shows how quickly the starting 10–15-kilovolt line was overcome: in 1898–1902. 35–40 kV transmission lines were mastered,
From the book History of Electrical Engineering the author Team of Authors11.4.1. STAGES OF DEVELOPMENT Information electronics is a combination of hardware and algorithms (methods of processing and converting information) that perform the functions of collecting, processing, storing, displaying information and its use in tasks
ANSWERS
Materials Science. Classification of metals. Atomic-crystalline structure of metals. Types of gratings and their characteristics.
2.1. Material science is a scientific discipline about the structure, properties and purpose of materials. The properties of technical materials are formed in the process of their manufacture. With the same chemical composition, but different manufacturing techniques, a different structure is formed, and, as a result, properties.
The purpose of materials science is to study the laws of formation of the structure and properties of materials by methods of their hardening for effective use in technology.
The main task of materials science is to establish the relationship between composition, structure and properties, to study the thermal, chemical-thermal treatment and other methods of hardening, to form knowledge about the properties of the main types of materials.
2.2. All metals are conventionally divided into black and non-ferrous. Ferrous metals usually have a dark gray color, high density (except alkaline), high melting point, relatively high hardness. Some of them (iron, titanium, cobalt, manganese, zirconium, uranium, etc.) possess polymorphism (allotropy). The most typical ferrous metal is iron.
Non-ferrous metals are red, yellow, white. They have great ductility, low hardness, low melting point. Tin is known to have polymorphism. A typical representative is copper.
Ferrous metals include:
- iron metals - iron, cobalt, nickel, manganese;
- refractory metals; have a melting point higher than that of iron, i.e. more than 15390С
Titanium, vanadium, chromium, zirconium, niobium, molybdenum, tungsten, technetium, hafnium, rhenium;
- uranium metals (actinides) - thorium, sea anemone, uranium, neptunium, plutonium, etc. (from 89 to 103 elements);
- rare earth metals (with 57 -71 elements), lanthanum, cerium, niodimum, etc.
- alkaline earth metals
Lithium, sodium, calcium, potassium, rubidium, strontium, cesium, barium, France, rhodium, scandium.
Non-ferrous metals include:
- lungs - beryllium, magnesium, aluminum;
- noble metals
Ruthenium, radium, palladium, osmium, iridium, platinum, gold, silver and semi-precious copper;
- fusible metals - zinc, cadmium, mercury, gallium, indium, waist, germanium, tin, lead, arsenic, antimony, bismuth.
Metals and alloys include substances obtained by powder metallurgy.
Classification of non-metallic materials:
- organic and inorganic polymers;
- plastics;
- composite materials;
- rubbers and rubber;
- adhesive materials and sealants;
- paintwork;
- graphite;
- glass;
- ceramics.
The state diagram of the system with the complete insolubility of the components in the solid state (with eutectic).
Figure 1 - State diagram of eutectic alloys
In these alloys, the components in the solid state are insoluble in each other and do not chemically interact.
Single phase areas of the diagram:
1) liquid L - above the line of liquidus DCE;
2) phase A - line 0FD;
3) phase B - line 100-G-E.
The characteristic point of the diagram is the triple point C, it corresponds to a eutectic alloy containing C "% B. The eutectic in these alloys consists of crystals A and B, its region in the diagram is the SS line." FCG line - eutectic transformation line: L eut -\u003e eut (A + B). The same line is solidus. The crystallization of the alloys of this system begins on the DCE line with the release of solid crystals of the component that is redundant with respect to the eutectic composition, and ends on the FCG line by the eutectic transformation.
Structural components of alloys (and their areas in the diagram):
1) crystals A - line 0FD;
2) crystals B - line 100-G-E;
3) eutectic crystals (eut (A + B)) - SS line. "
The process of graphitization during annealing of white cast iron.
Rockwell Method (GOST 9013)
It is based on pressing into the surface of the tip under a certain load (Fig. 7.1 b)
Indenter for soft materials (up to HB 230) - a steel ball with a diameter of 1/16 ”(Ø1.6 mm), for harder materials - a diamond cone.
Loading is carried out in two stages. First, a preload (10 kf) is applied to tightly touch the tip with the sample. Then the main load P 1 is applied, for some time the general work load P is applied. After removing the main load, the hardness value is determined by the depth of the residual indentation of the tip h under load.
Three hardness scales A, B, C are used depending on the nature of the material.
Hardness is determined by the size of the imprint (Fig. 7.1 c).
A diamond tetrahedral pyramid is used as an indenter. With an angle at the apex of 136º.
Hardness is calculated as the ratio of the applied load P to the surface area of \u200b\u200bthe print F:
The load P is 5 ... 100 kgf. Fingerprint diagonal dmeasured using a microscope mounted on the device.
The advantage of this method is that it is possible to measure the hardness of any materials, thin products, surface layers. High accuracy and sensitivity of the method.
Microhardness Method used to determine the hardness of the individual structural components and phases of the alloy, very thin surface layers (hundredths of a millimeter).
Similar to Vickers method. The indenter is a pyramid of smaller sizes, the indentation loads P are 5 ... 500 gs
Scratch method.
With a diamond cone, pyramid or ball, a scratch is applied, which is a measure. When scratching other materials and comparing them with a measure, they judge the hardness of the material.
A 10 mm wide scratch can be applied under a certain load. Observe the magnitude of the load that gives this width.
Dynamic method (Shore)
The ball is thrown to the surface from a given height, it bounces a certain amount. The larger the rebound, the harder the material.
As a result of dynamic tests for impact bending of special notched specimens (GOST 9454), the viscosity of the materials is evaluated and their tendency to transition from a viscous to a brittle state is established.
Technological properties
Technological properties characterize the ability of the material to undergo various methods of cold and hot processing.
1. Foundry properties.
Characterize the ability of the material to obtain high-quality castings from it.
Fluid flow - the ability of molten metal to fill the mold.
Shrinkage (linear and volumetric) - characterizes the ability of the material to change its linear dimensions and volume during solidification and cooling. To prevent linear shrinkage when creating models using non-standard meters taking into account the shrinkage of a certain metal ...
Segregation - heterogeneity of the chemical composition by volume.
2. The ability of the material to pressure treatment.
This is the ability of a material to change its size and shape under the influence of external loads without collapsing.
It is controlled as a result of technological tests conducted under conditions as close as possible to production.
The sheet material is tested for bending and drawing a spherical hole. The wire is tested for bending, twisting, winding. Pipes are tested for distribution, flattening to a certain height and bending.
The criterion for the suitability of the material is the absence of defects after testing.
3. Weldability.
This is the ability of the material to form permanent compounds of the required quality. Evaluated by the quality of the weld.
4. The ability to process by cutting.
It characterizes the ability of the material to be machined by various cutting tools. Evaluated by the durability of the tool and the quality of the surface layer.
Operational properties
Operational properties characterize the ability of the material to work in specific conditions.
Wear resistance - the ability of the material to resist surface destruction under the action of external friction.
Corrosion resistance - the ability of the material to resist the action of aggressive acidic, alkaline environments.
Heat resistance - this is the ability of a material to resist oxidation in a gaseous environment at high temperature.
Heat resistance - this is the ability of a material to maintain its properties at high temperatures.
Cold resistance - the ability of the material to maintain plastic properties at low temperatures.
Anti-friction - the ability of the material to break in to another material.
These properties are determined by special tests depending on the working conditions of the products.
When choosing a material to create a structure, it is necessary to fully take into account the mechanical, technological and operational properties.
The formation of austenite and its grain growth upon heating. Overheating and burnout.
The formation of austenite when heated
State diagram Fe - C
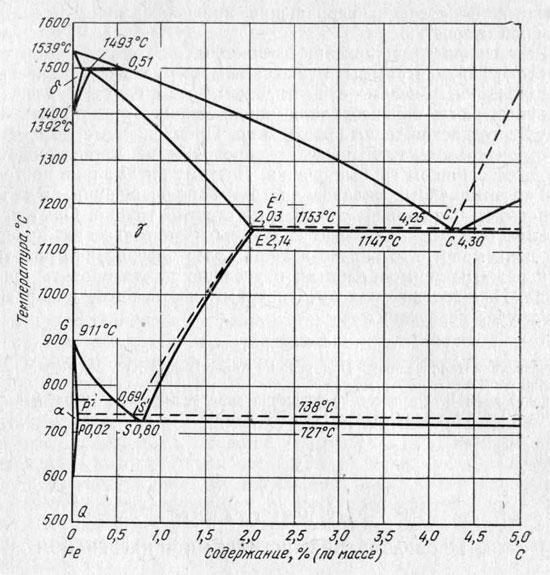
The transition of perlite to austenite, its kinetics obey the basic laws of phase transformations that occur during heating.
It was experimentally established that austenite nuclei arise at the boundaries of ferrite with cementite. The initial stages of formation of austenite nuclei have not been experimentally studied, and there are only assumptions about them. The conversion of α o.c.c. → γ g.c.c. in pure iron is only possible at temperatures not lower than 911 ° C. If ferrite is in contact with cementite, then, in accordance with the state diagram, the α - γ transformation should occur at temperatures starting from 727 ° С. Austenite at a temperature slightly above point A 1 contains about 0.8% C, while ferrite in steel contains hundredths of a percent carbon.
How, then, does the phase phase arise with the city center. K. lattice and relatively high carbon content?
Most hypotheses of the origin of austenite come from fluctuation representations, and two extreme cases are formally considered. First, one can imagine that the basis for the nucleation of austenite are concentration fluctuations. Inside ferrite, the probability of the formation of a significant number of fluctuation regions of critical size is negligible, since there are very few carbon atoms. At the boundary of ferrite with cementite between the phases, there is a continuous exchange of atoms (dynamic equilibrium) and in the boundary layer (ferrite is much more likely to fluctuate in the occurrence of sections of a critical size with a concentration of about 0.8% C.
Such sites, at any smallest overheating above point A 1, undergo polymorphic α - γ transformation of the solid solution and become stable centers of growth of austenitic grains. Below point A 1, similar sites in ferrite can also arise, but they do not turn into stable centers of austenite growth, since the γ-lattice is thermodynamically unstable here.
Another assumption is that when austenite nucleates, it is not concentration fluctuations that are primary, but the fluctuation rearrangement of the lattice. Inside ferrite, regions with a γ-lattice of fluctuational origin appear and disappear, and carbon from carbide enters these regions at temperatures above A 1 at temperatures above A 1 and, if they are of critical size, they become stable centers of growth of austenite.
22.2.
If you heat the metal to the upper critical point and continue to raise the temperature, then, examining the metal under a microscope, you can detect the growth of its grains.
The higher the temperature, the more vigorously the grain grows and the larger they are, the longer the process of heating to a given temperature. A metal having highly coarse grains is called superheated metal.
In the forging process, strongly overheated metal gives flaws and cracks, especially in the corners of an ingot or billet, and in a fracture has a greatly enlarged structure, which can be relatively easily observed with a simple eye. Overheating depends on two factors: temperature and heating time.
From the practice of forging furnaces it is known that if an ingot or billet is kept in a furnace at a high temperature (for example, in the welding part of a method furnace) more than usual, then when forging such an ingot or billet, flaws result from overheating. On the contrary, an ingot located in the furnace at the same temperature, but for a shorter time, is forged quite normally.
Thus, overheating of the metal is possible at any temperature exceeding the critical point, but the amount of overheating at this temperature depends on the exposure time.
Overheated metal can be corrected by subsequent annealing, i.e., by slow heating to a temperature 10-30 above the point, and subsequent slow cooling.
If the heated metal is left in the furnace for a long time at a high temperature, then it will burn out. The burning occurs because the oxygen in the furnace gases penetrates deep into the metal from the surface, the grain boundaries of the metal are oxidized, and the substance formed between the large grains melts. As a result, liquid films form between the grains of the metal, the bond between the grains is broken, and the metal becomes fragile, large cracks appear on the workpiece, and it breaks up into pieces. Further heating leads to the melting or destruction of individual sections of the workpiece. The burning depends mainly on the heating temperature, the composition of the furnace gases and the heating time of the metal at high temperatures.
The burnt metal cannot be fixed, the billet is usually rejected, and the preserved metal can only be used by smelting in an open-hearth furnace.
To prevent burnout of the metal, it is necessary to observe the following basic conditions when heating:
1. Burn fuel with the lowest coefficient of excess air so that there is no free oxygen in the furnace gases.
2. Do not load blanks under the furnace in bulk, but arrange them so that they are washed with furnace gases, if possible, and torch burners or nozzles would not (lick) the surface of the heated blanks.
3. You can load so much metal into the furnace that the forging unit can forge it in the time it takes to heat the workpiece to the forging temperature. It is better to load the furnace by the piece method, that is, one or two heated billets are discharged from the furnace, and cold billets are fed into their place, etc. In case of piece loading, the length of time the metal stays at high temperatures will be what it takes to heat it. And this will make it possible to avoid overheating and burnout of the metal.
Self tempering.
Heated products are placed in a cooling medium and kept until incomplete cooling. After removing the product, its surface layers are reheated due to internal heat to the required temperature, that is, self-tempering is carried out (see. Steel tempering). It is used for products that must combine high hardness on the surface and high viscosity in the core (impact tools: hammers, chisels).
The process technology is as follows: Loading parts into a steel box with an airtight sand shutter. The parts are laid in such a way that they are covered with a carburetor on all sides, not in contact with each other and the walls of the box. Further, the box is hermetically sealed with a sand shutter or covered with refractory clay and loaded into the furnace.
Standard mode: 900-950 degrees, 1 hour exposure (after warming up the box) per 0.1 mm of the thickness of the cemented layer. to obtain 1 mm layer - exposure for 10 hours.
In the "accelerated" mode, cementation is performed at 980 degrees. Exposure is halved and it takes 5 hours to get a 1 mm layer. But at the same time, a cementite mesh is formed, which will have to be removed by multiple normalization of the metal
This process is carried out in an atmosphere of gases containing carbon. Gas cementation has several advantages compared to cementation in a solid carburetor, therefore it is widely used in factories manufacturing parts in bulk.
In the case of gas cementation, you can get a given concentration of carbon in the layer; the duration of the process is reduced, since there is no need to warm up boxes filled with a low-heat carburetor; the possibility of complete mechanization and automation of processes is ensured and the subsequent thermal treatment of parts is greatly simplified, since hardening can be carried out directly from the cementation furnace.
High speed steels
High-speed steels are widely used for the manufacture of cutting tools operating under conditions of significant force loading and heating (up to 600-640 ° C) of cutting edges. This group of steels includes high-alloyed tungsten together with other carbide-forming elements (molybdenum, chromium, vanadium), which acquire high hardness, strength, heat and wear resistance as a result of double hardening: a) martensitic during hardening; b) dispersion hardening at a relatively high tempering (500-620 ° C), which causes the release of hardening phases.
High-speed steels are marked with the letter "P" (rapid - fast) and a number showing the average content of W, as well as subsequent letters and numbers indicating other alloying elements and their quantity, as in the standard marking of alloyed steels. Carbon and chromium do not indicate high-speed steels (their mass fraction is 1% and 4%, respectively), as well as molybdenum up to 1% inclusive and vanadium in steels P18, P9, P9K5, P6M5, etc.
The chemical composition of high-speed steels is given in table. 6.7.
According to the main properties, high-speed steels are divided into five subgroups: 1) steel of moderate heat resistance (type P9, P6M5); 2) increased wear resistance (type R12F3, R6M5F3); 3) increased heat resistance (type P6M5K5, P9K5); 4) high wear and heat resistance (type R18K5F2); 5) high hardness and heat resistance with improved grindability (type P9M4K8, V11M7K23).
However, these steels have many common characteristics. Therefore, to simplify the consideration of structural features, properties and heat treatment modes, they can be divided into three groups according to processing performance:
· Steel of normal performance (steel of moderate heat resistance);
· Steel of increased productivity (steel of increased heat and wear resistance);
· High-performance steels (steels of high heat and wear resistance).
· The structure of steels with carbide hardening (steel type "P") is approximately the same for all groups. After the final heat treatment (quenching + tempering), their structure consists of martensite with the release of dispersed particles of alloyed carbides mainly of the type M 6 C and MS. Such a structure provides heat resistance of the tool up to 600–640 ° С.
· The highest heat resistance (up to 700–720 ° С) are observed in highly alloyed Fe-Co-W-Mo alloys with intermetallic hardening (grades V4M12K23 and V11M7K23). After the final heat treatment, the structure of these alloys consists of carbon-free (or low-carbon) martensite with a low hardness (30–40 HRC E) and finely divided intermetallic compounds (Fe, Co) 7 (W, Mo) 6, Fe 3 W 2 (Fe 3 Mo 2) , (Fe, Co, Ni) 7 (W, Mo) 6.
· High hardness (HRC E 68–70) and heat resistance (720 ° C) are ensured by: a) higher temperatures (900–950 ° C) of the onset of phase transformations, which is 100 ° C higher than that of steel with carbide hardening; b) large quantities of hardening phases, characterized by high dispersion (up to 2-3 microns) and uniform distribution in the main matrix.
· High-speed steels belong to the ledeburite (carbide) class and their structure is approximately the same. Ingots of these steels contain carbide eutectic in the form of a grid along the boundaries of austenitic grains (Fig. 6.1, a), which sharply reduces the usual mechanical properties, especially ductility. In the process of hot pressure treatment (forging, rolling), the carbide eutectic is crushed and the crushed carbides are more evenly distributed in the main matrix (Fig. 6.1, b).
· After rolling or forging, high speed steels are subjected to isothermal annealing to reduce hardness and facilitate machining. Steel is maintained at 800–850 ° С until austenite is completely converted into a pearlite-sorbitol structure with excess carbides
Heat treatment.High hardness and heat resistance with satisfactory strength and toughness tools from high-speed steels acquire after hardening and repeated tempering.
Quenching . When heating under quenching, it is necessary to ensure maximum dissolution in austenite of insoluble carbides of tungsten, molybdenum and vanadium. Such a structure increases hardenability and makes it possible to obtain highly alloyed martensite with high heat resistance after quenching. Therefore, the quenching temperature is very high and amounts to 1200–1300 ° С
To prevent cracking and deformation of the tool due to the low thermal conductivity of the steels, quenching is carried out with one or two heatings in molten salts: the first at 400–500 ° С, the second at 800–850 ° С. The final heating is also carried out in a salt bath (BaCl 2) with a very low shutter speed at T c: 10–12 s per 1 mm of tool thickness made of “P” steels and 30–60 s for steel of type B11M7K23. This avoids the growth of austenitic grain (no larger than No. 10), oxidation and decarburization.
Tools of a simple form are quenched in oil, and complex ones in solutions of salts (KNO 3) at 250–400 ° С.
After quenching, the structure of high-speed steel (Fig. 6.1, c) consists of highly alloyed martensite containing 0.3–0.4% C, not dissolved during heating of excess carbides, and about 20–30% of residual austenite. The latter reduces the hardness, cutting properties of the tool, degrades sandability, and its presence is undesirable.
Vacation With multiple tempering, dispersed carbides precipitate from residual austenite, austenite doping decreases, and it undergoes a martensitic transformation. Usually, triple tempering is used at 550–570 ° С for 45–60 min. The heat treatment mode of the tool made of high-speed steel P18 is shown in Fig. 6.2. The number of leaves can be reduced by cold treatment after quenching, which reduces the content of residual austenite. Cold processing tools are subjected to a relatively simple form. Hardness after hardening HRC E 62–63, and after tempering it increases to HRC E 63–65.
Surface treatment. To further increase the hardness, wear resistance and corrosion resistance of the surface layer of cutting tools, technological operations such as cyanidation, nitriding, sulfidation, steam treatment and other surface hardening technologies are used. They are performed after the final heat treatment, grinding and sharpening of tools.
ianization is carried out at 550–570 ° С for 5–30 min in liquid media and 1.5–3.0 h in a gas atmosphere. For liquid cyanidation, baths with molten NaCN (90 or 50%), Na 2 CO 3, NaOH (KOH) are used. Gas cyanidation is carried out in a mixture of ammonia and carburizing gas.
Nitriding of the instruments is carried out at 550–660 ° С for a duration of 10–40 min in an ammonia atmosphere. Gas nitriding is also carried out in a mixture of 20% ammonia and 80% nitrogen; the latter is preferable, since in this case less fragility of the layer is ensured.
Sulphidation is carried out at 450–560 ° С, lasting from 45 minutes to 3.0 hours in liquid melts, for example, 17% NaCl, 25% BaCl 2, 38% CaCl 2, 3-4% K 4 Fe (CN) 6, which add sulfur-containing compounds FeS, Na 2 SO 4, KCNS.
When steaming, the tools are placed in a sealed oven and kept at 300–350 ° С under a pressure of 1-3 MPa for 20–30 min to remove air. Then, the temperature rises to 550–570 ° С, holding is carried out for 30–60 min, cooling in the atmosphere of steam to 300–350 ° С, after which the steam supply stops. Cooling ends in an oven or in air, then the instrument is immediately rinsed in hot spindle oil.
Application. A competent choice of the steel grade for a particular tool, depending on the conditions of its operation and the material being processed, makes it possible to maximize the use of the properties properties of the selected steel and, as a result, rationally consume alloying materials, as well as determine the need for certain coatings, surfacing and other surface hardening methods. In the table. 6.9. recommended areas of application of the most common brands of high-speed steels are presented depending on the types of processed materials and types of processing. This approach to the selection of tool steels for any purpose helps to increase both productivity and production efficiency.
ANSWERS
The role of materials in modern technology. On the history of the development of materials science as a science
Material science can be attributed to those branches of physics and chemistry that study the properties of materials. In addition, this science uses a number of methods to study the structure of materials. In the manufacture of high-tech products in industry, especially when working with micro- and nanoscale objects, it is necessary to know in detail the characteristics, properties and structure of materials. To solve these problems and called science - materials science.
The beginning of the development of materials science can be considered the moment when a person first began to choose what to take in his hand - a stick or a stone, that is, the birth of materials science coincides with the beginning of the Stone Age.
Therefore, material science is one of the oldest forms of applied science, which, together with humanity, has come a long way from primitive stone processing and the manufacture of simple ceramics to modern superpopular nanotechnologies. For a long time, metallurgy and metal science prevailed in materials science, that is, the science of materials was equated in fact with the science of metals.
Modern materials science is also based on metal science, however, in addition to metals and alloys, material science studies many other diverse materials, both by purpose (plastics, semiconductors, biomaterials) and composition (carbon materials, ceramics, polymers, etc.)
